Evaporation looks straightforward: heat water until it turns to vapor, then collect what’s left behind. But there’s more happening beneath the surface. Three physical factors drive the entire process: temperature, airflow, and surface area. Each one matters on its own, but it’s how they interact that determines whether an evaporator runs efficiently or wastes energy. Knowing what’s actually happening inside the tank helps explain why some systems deliver results while others fall short.
Temperature: Driving the Change from Liquid to Vapor
 Heat gets things moving. When wastewater absorbs enough thermal energy, water molecules break free from the liquid and escape as vapor. Pure water boils at 212°F, but wastewater isn’t pure. Dissolved salts, oils, and other contaminants raise the boiling point slightly, sometimes just a few degrees, sometimes more depending on what’s in the mix. That’s why operators can’t just set a dial and walk away. The system needs adjustment until the water reaches a steady, rolling boil that holds consistent over time.
Heat gets things moving. When wastewater absorbs enough thermal energy, water molecules break free from the liquid and escape as vapor. Pure water boils at 212°F, but wastewater isn’t pure. Dissolved salts, oils, and other contaminants raise the boiling point slightly, sometimes just a few degrees, sometimes more depending on what’s in the mix. That’s why operators can’t just set a dial and walk away. The system needs adjustment until the water reaches a steady, rolling boil that holds consistent over time.
Airflow: Carrying Vapor Away
Heating the water is only half the job. Once molecules vaporize, they hover above the liquid surface. Without airflow, that vapor builds up and creates a humid layer that chokes off further evaporation. It’s like trying to dry laundry in a closed closet: nothing moves, so nothing dries. Airflow sweeps away the moisture and keeps the process running. Well-designed systems use fans or vents positioned to maintain continuous air movement, so evaporation doesn’t stall even after hours of operation.
Surface Area: More Exposure Means More Evaporation
The larger the liquid surface, the more water molecules can escape at once. Picture a shallow puddle versus a deep bucket: the puddle dries faster because more of the water touches open air. Some evaporators use wide, shallow tanks to maximize this effect. Others incorporate agitators or spray nozzles that break up the liquid and increase exposure. Either way, expanding surface area speeds up evaporation without cranking up the heat, which saves energy and reduces wear on equipment.
Balancing the Three Factors
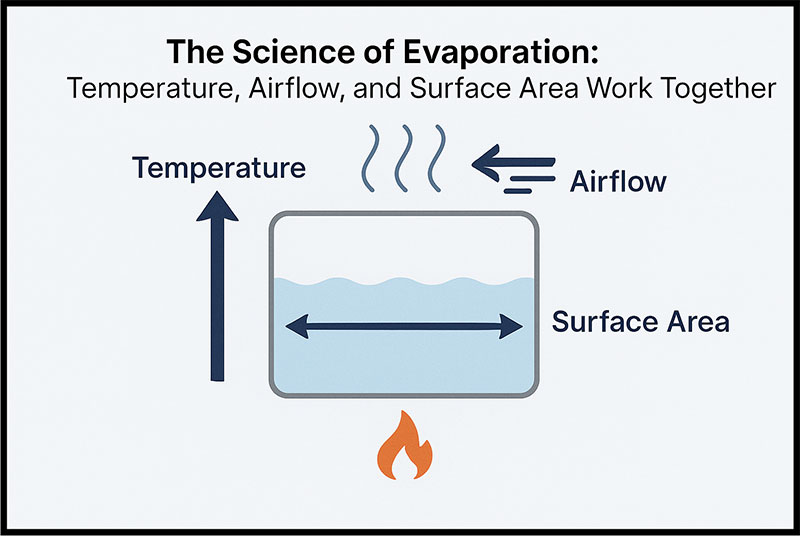 Temperature, airflow, and surface area don’t operate in isolation. Push one too hard and the others can’t keep up. Crank the heat without adequate airflow, and vapor just sits there doing nothing. Spread wastewater across a huge surface but skimp on heat, and evaporation crawls. The systems that work best are the ones engineered with all three elements in mind: enough heat to vaporize water, enough airflow to clear it out, and enough surface area to keep the process moving without burning through fuel or electricity.
Temperature, airflow, and surface area don’t operate in isolation. Push one too hard and the others can’t keep up. Crank the heat without adequate airflow, and vapor just sits there doing nothing. Spread wastewater across a huge surface but skimp on heat, and evaporation crawls. The systems that work best are the ones engineered with all three elements in mind: enough heat to vaporize water, enough airflow to clear it out, and enough surface area to keep the process moving without burning through fuel or electricity.
Conclusion
Evaporation isn’t magic, and it’s not as simple as boiling a pot on the stove. It’s a controlled process where temperature, airflow, and surface area have to pull together. Facilities that grasp this can spot bad designs before they buy, troubleshoot problems when performance drops, and choose equipment that actually reduces wastewater volume without surprise costs down the line. Understanding the science means making smarter decisions, and that pays off year after year.
David N Lyman, a mechanical engineer with 40 years of industrial experience. Currently the president of RunDry Evaporators™.
